Cool Kitchen Pantry Science Experiments
With common household supplies and a few ordinary mason jars, kids can learn about science and experience the fun of discovery — no science lab required.

Science, for those of you now playing the role of teacher to stir-crazy kids, is much more than just a subject taught in school. It’s a process we humans invented to help us sort facts from fantasy, and truth from superstition.
To show your kids the value of science, and to experience the fun of discovery while we all keep our social distance, try these easy experiments. All you’ll need is some common household supplies and some ordinary mason jars.
Lava Lamp 2.0
Bubbles and blobs make for a mesmerizing reaction.
If you ask your grandparents about the original lava lamp, they just might get a faraway look in their eyes thinking about the groovy days of the 1960s. But if you want to really blow their minds, show them this simplified version and explain the science behind it. It all comes down to a scientific principle demonstrated every day by the salad dressing in your fridge: oil and water don’t mix.
What You’ll Need
- Pint-size (or larger) mason jar with two-piece lid*
- Water
- Food coloring
- Vegetable oil
- Effervescent antacid tablets (such as Alka–Seltzer)
*A larger jar gives more dramatic results.
Directions
- Fill the jar about a quarter full with water. Stir in 10 or so drops of food coloring.
- Add twice as much oil as water, so the jar is three-quarters full.
- Drop in half of an antacid tablet and observe the reaction.

What to Watch For
The tablet should start to bubble, causing blobs of colored water to rise up into the oil. Once the initial bubbling slows down, watch as smaller bubbles continue to rise and fall, or add more antacid, broken into smaller chunks.
When you are done, let the jar sit uncovered for a while so all the gas can escape. (Trapping that gas in the jar could cause it to crack!) Store the jar with the lid on, and keep some antacid tablets handy, so you can use your lamp on another groovy day.
What’s Going On
The original lava lamp used heat to send mesmerizing blobs of wax rising and falling. Here, we use the gassy fizz from an effervescent antacid tablet. The oil is less dense than the colored water so it floats in a layer on top. The blobs of water are carried to the surface by the rising carbon dioxide bubbles, and then sink back down when the bubbles reach the top.
A material that bubbles or foams from escaping gas is called effervescent. Antacid tablets are effervescent in the same way that baking soda is when you mix it with an acid such as vinegar. In fact, the tablets actually contain baking soda (sodium bicarbonate) along with citric acid, so when the tablet is dissolved in water, you see a similar reaction: lots of bubbles of carbon dioxide gas.
Cornstarch Quicksand
Make a solid liquid. Or is it a liquid solid?

Experts will tell you that if you ever happen to be trapped in quicksand, you shouldn’t panic and thrash about, which will only sink you deeper. Instead, you should lie back so you float on the watery sand, then slowly — slowly! — paddle your way to solid ground. So now you know what to do if you happen to fall into a huge vat of this cornstarch and water mixture. Like quicksand, it’s a mysterious ooze, somewhere between a liquid and a solid, and it’s full of surprises.
What You’ll Need
- Any size mason jar with two-piece lid
- Water
- Cornstarch
- Wooden spoon or other stirrer
- Shallow pan
Directions
- Using a formula of one part water to two parts cornstarch, mix up however much quicksand you want. (For a large batch, use one cup of water and two cups of cornstarch in a quart jar.) Use a stiff wooden spoon to stir the mixture. It will be hard to mix together, but keep at it! Eventually, it will form a thick paste.
- Pour the quicksand into a shallow pan and walk your fingers across it. When they sink in, try to pull them out fast, like a panicked person might, and see what happens. Lift some mixture in your hands and squeeze it. Release your grip and see what happens.
NOTE: Your quicksand will last a while stored in the jar, but when you want to get rid of it, don’t pour it down the drain (it can cause a clog). Instead, throw it away or compost it.

What to Watch For
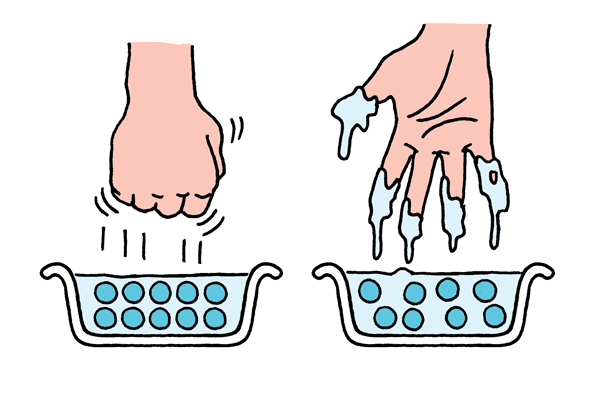
The mixture will feel stiff if you stir quickly, but more loose if you stir slowly. Tap your stirrer on the mixture’s surface. It feels solid. Scoop the mixture up with the stirrer. It flows off like a liquid!
What’s Going On
You’ve created a colloidal solution, a mixture made up of very tiny particles suspended in water. Quicksand, which is just sand suspended in water, is very similar. These types of mixtures can act like both solids and liquids, depending on what you are doing to them.
The eighteenth-century scientist Sir Isaac Newton (of “law of gravity” fame) studied fluids and came up with rules for how the ideal ones should act. Colloidal solutions are so weird, they are referred to as non-Newtonian fluids.
Walking Water Colors
Make colored water move from jar to jar.

Sometimes science seems like magic, as it does in this experiment. How can water flow uphill and over the edge of a jar? What happened to gravity? The answer is capillary action, which you’ve experienced if you’ve ever accidentally stepped in a deep puddle and later felt the water slowly climbing up your pants. Here, that same force moves the colored water from one jar to another. As a bonus, you get to see another bit of science magic: how three colors can become six.
What You’ll Need
- 7 half-pint (or pint-size) mason jars
- Food coloring
- Water
- Paper towels
Directions
- Fill four jars with water. With the food coloring, color two of them red, one blue, and one yellow. We used 10 drops in each.
- Arrange the jars in the following pattern: red, empty, blue, empty, yellow, empty, red.
- Roll six sheets of paper towel into wicks about an inch wide, and then fold them in half. Carefully place one end of each wick in a jar, as shown.
What to Watch For
After a few minutes, the water from the full jars will climb up the wick and start flowing into the empty jars. Watch what happens when the colors start to mix. What do you notice about the amount of water in each jar when the process stops?
What’s Going On
The water rises up the paper towel because of capillary action, the same process that explains how the hairs of a paintbrush soak up paint, how plants draw water up through their stems, and how a polypropylene sports shirt wicks sweat away from your skin. Water wants to stick to things, a force called adhesion. It also wants to stick to itself, a force called cohesion.
Those forces make water flow into narrow spaces (such as into the air pockets of a paper towel) and are so strong that they can overcome gravity for a short distance. Once the water wicks its way over the high point of the paper towel, gravity takes over and the water flows down the other side, stopping when the level in each jar is the same.
Red, blue, and yellow are called the primary colors because with careful mixing of just those three, you can create all the other shades. That’s how your computer printer can create every color from just three inks (with ink, blue is called cyan, and red is magenta). Experiment with how you set up your wicking jars to see which new colors you can create!
EXCERPTED FROM MASON JAR SCIENCE © BY JONATHAN ADOLPH. ALL RIGHTS RESERVED.
Nautilus Book Awards Silver Winner
Heatproof, transparent, and durable, the mason jar is a science lab just waiting to be discovered. Unlock its potential with 40 dynamic experiments for budding scientists ages 8 and up. Using just a jar and a few ordinary household items, children learn to create miniature clouds, tiny tornadoes, small stalactites, and, of course, great goo and super slime! With a little ingenuity, the jar can be converted into a lava lamp, a water prism, a balloon barometer, and a compass. Each fun-packed project offers small-scale ways to illustrate the big-picture principles of chemistry, botany, biology, physics, and more.