Bonus Activity: Make SLIME! and STEM Like a Girl
 Explore polymer chemistry and non-Newtonian fluids by making slime.
Explore polymer chemistry and non-Newtonian fluids by making slime.
Materials You’ll Need:
- Clear craft glue
- Baking soda
- Contact lens solution containing boric acid (for example: Re-Nu® Advanced Formula)
- Decorative mix ins such as glitter, beads, mini pom-poms
- Disposable plastic cup
- Spoon or craft stick
- Measuring spoons
Method
- Pour 5 oz of glue (poly-vinyl acetate) into a plastic cup.
Add ½ teaspoon of baking soda and stir with a spoon or craft stick.
Add in any mix-ins you’d like to decorate your slime.
Add 1 teaspoon of contact lens solution containing boric acid and stir.
- What do you observe?
- Depending on how “slimy” you want it, another ½ teaspoon of contact lens solution and stir. Go slow! You can always add more. More contact lens solution will result in a stiffer, more crosslinked, slime.
- Stretch your slime slowly and observe how it behaves. What happens if you pull it fast instead?
STEM Application
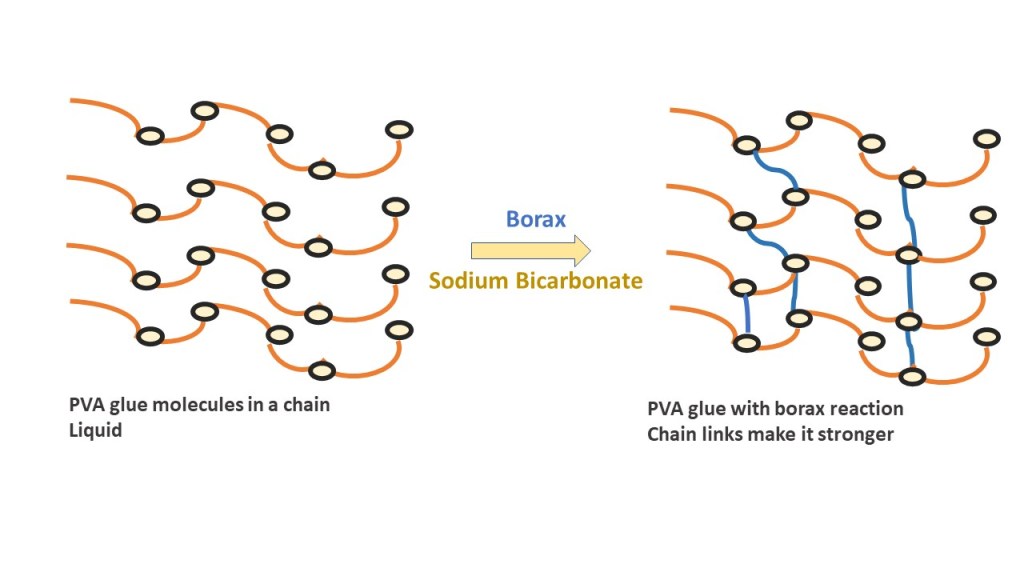
Glue is made up of poly-vinyl acetate (PVA) molecules which bond together, or crosslink, to form a polymer chain. In order to do this, the PVA needs to be activated to start the chemical reaction. Borate ions in the contact lens solution with the help of baking soda (sodium bicarbonate) act as the activators to start the crosslinking process. Too few boron molecules and the PVA won’t crosslink to form slime. Too many activators and your slime will become too stiff.
Once the slime is formed, it acts as a non-Newtonian fluid which means it can behave like a fluid AND a solid depending on the pressure applied. These non-traditional materials change how they flow under stress. When you apply a hard pressure by quickly pulling or poking the slime, it doesn’t splash like a true liquid would. Instead, it breaks apart more like a solid because the crosslinked PVA particles are forced together. When you slowly allow it to stretch out, the particles have time to move apart and it flows more like a liquid.
Want More? Check out Sarah Foster’s STEM Like a Girl!
