By clicking “Accept,” you agree to the use of cookies and similar technologies on your device as set forth in our Cookie Policy and our Privacy Policy. Please note that certain cookies are essential for this website to function properly and do not require user consent to be deployed.
Cascadia Revealed
A Guide to the Plants, Animals, and Geology of the Pacific Northwest Mountains
Contributors
Formats and Prices
- On Sale
- May 11, 2021
- Page Count
- 584 pages
- Publisher
- Timber Press
- ISBN-13
- 9781643261010
Price
$29.99Price
$39.99 CADFormat
Format:
- Trade Paperback $29.99 $39.99 CAD
- ebook $13.99 $17.99 CAD
This item is a preorder. Your payment method will be charged immediately, and the product is expected to ship on or around May 11, 2021. This date is subject to change due to shipping delays beyond our control.
Buy from Other Retailers:
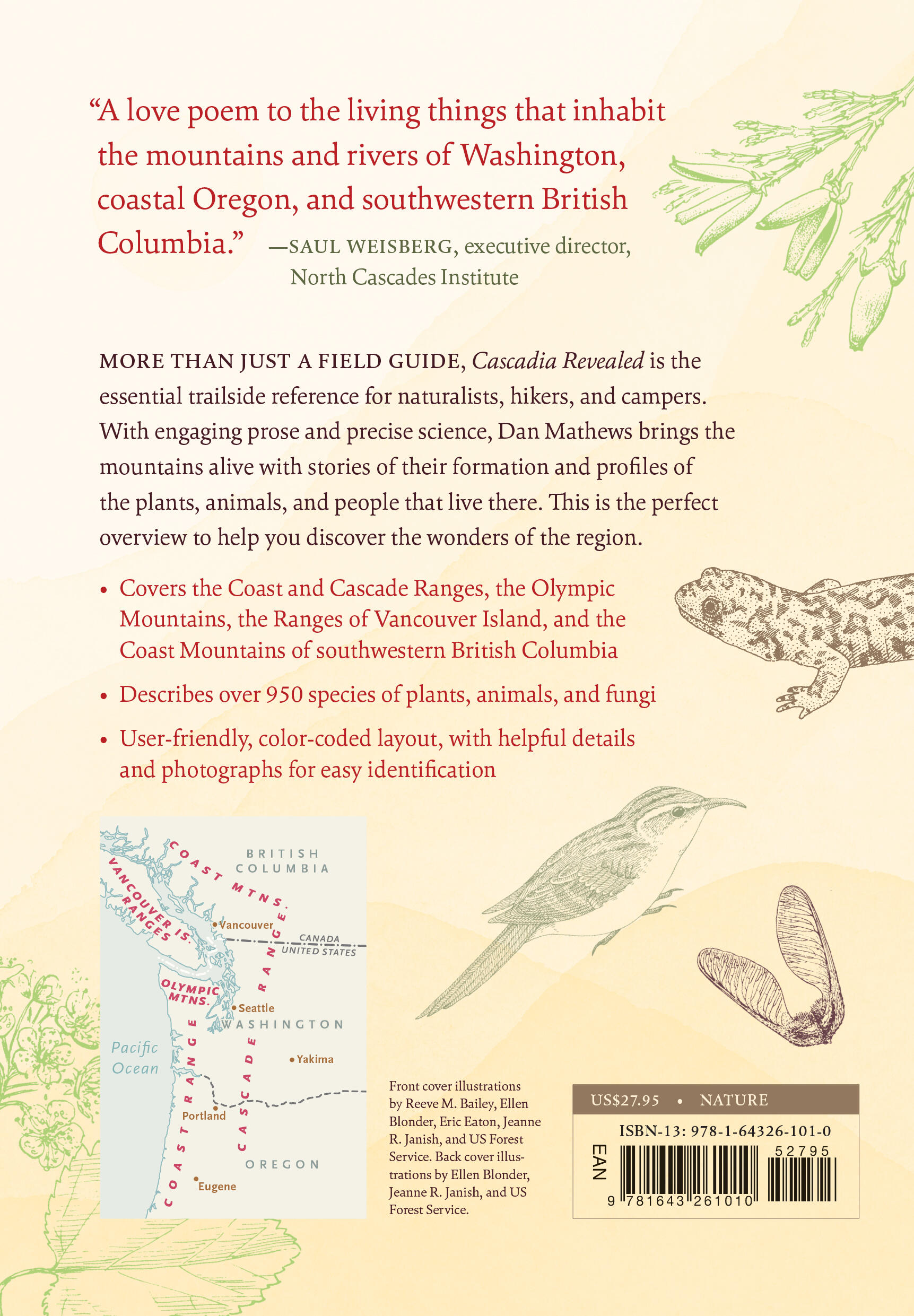
“A love poem to the living things that inhabit the mountains and rivers of Washington, coastal Oregon, and southwestern British Columbia.” —Saul Weisberg, executive director, North Cascades Institute
More than just a field guide, Cascadia Revealed is the essential trailside reference for naturalists, hikers, and campers. With engaging prose and precise science, Dan Mathews brings the mountains alive with stories of their formation and profiles of the plants, animals, and people that live there. This is the perfect overview to help you discover the wonders of the region.
- Covers the Coast and Cascade Ranges, the Olympic Mountains, the Ranges of Vancouver Island, and the Coast Mountains of southwestern British Columbia
- Describes more than 950 species of plants and animals
- User-friendly, color-coded layout, with helpful keys for easy identification
Newsletter Signup
By clicking ‘Sign Up,’ I acknowledge that I have read and agree to Hachette Book Group’s Privacy Policy and Terms of Use